

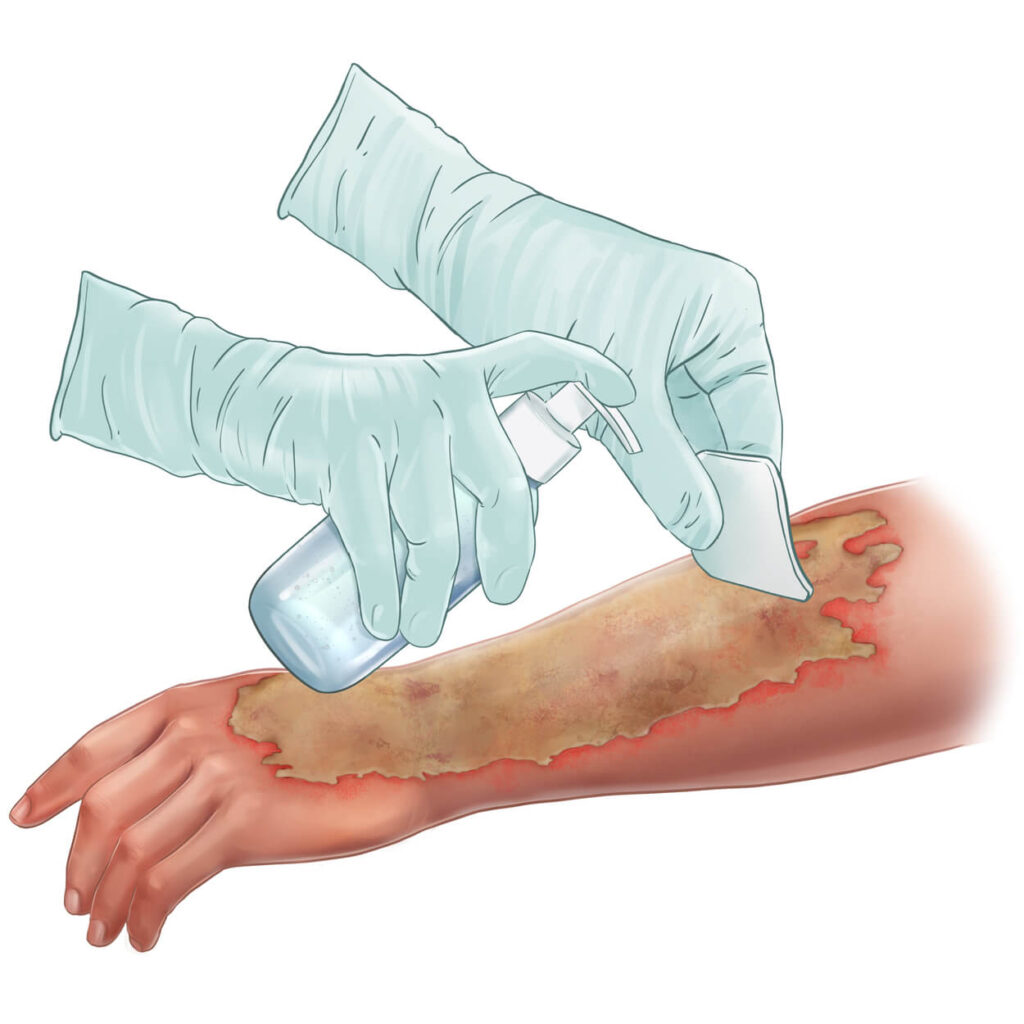
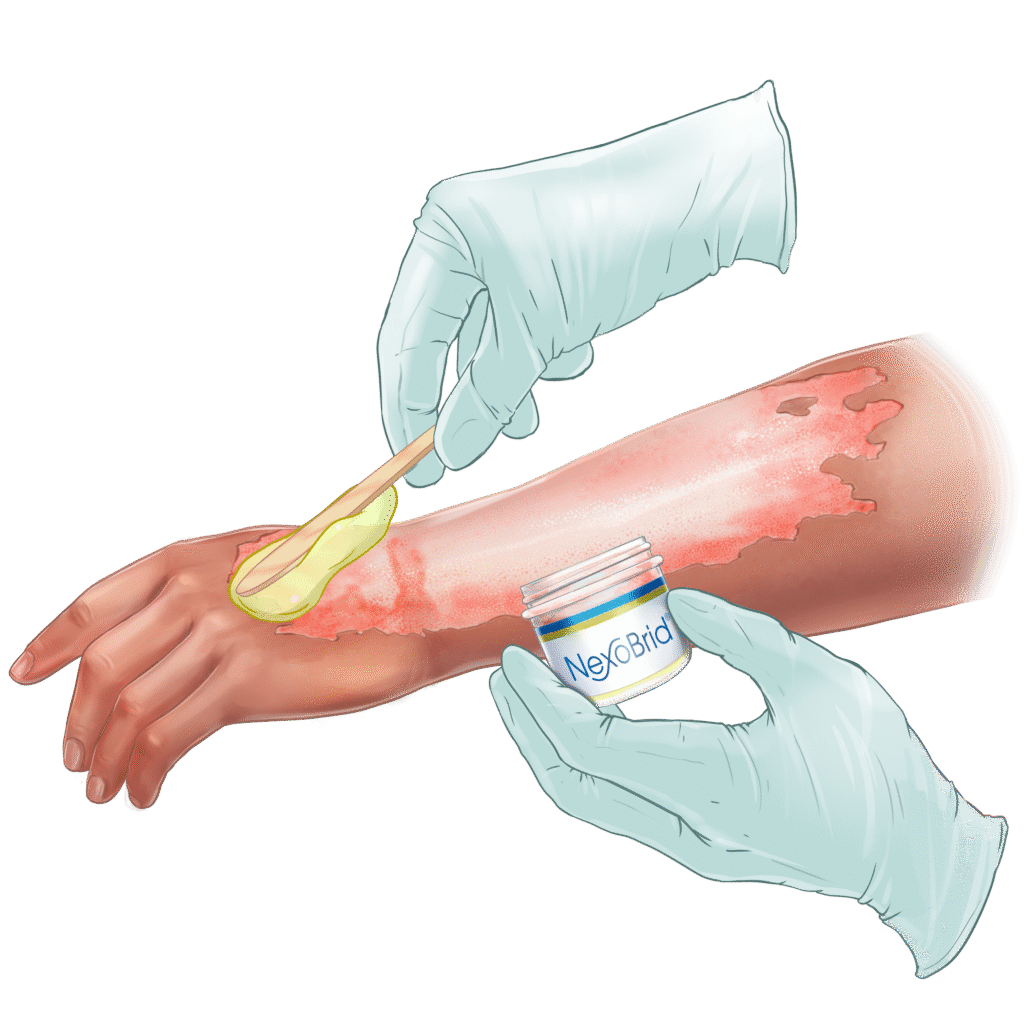
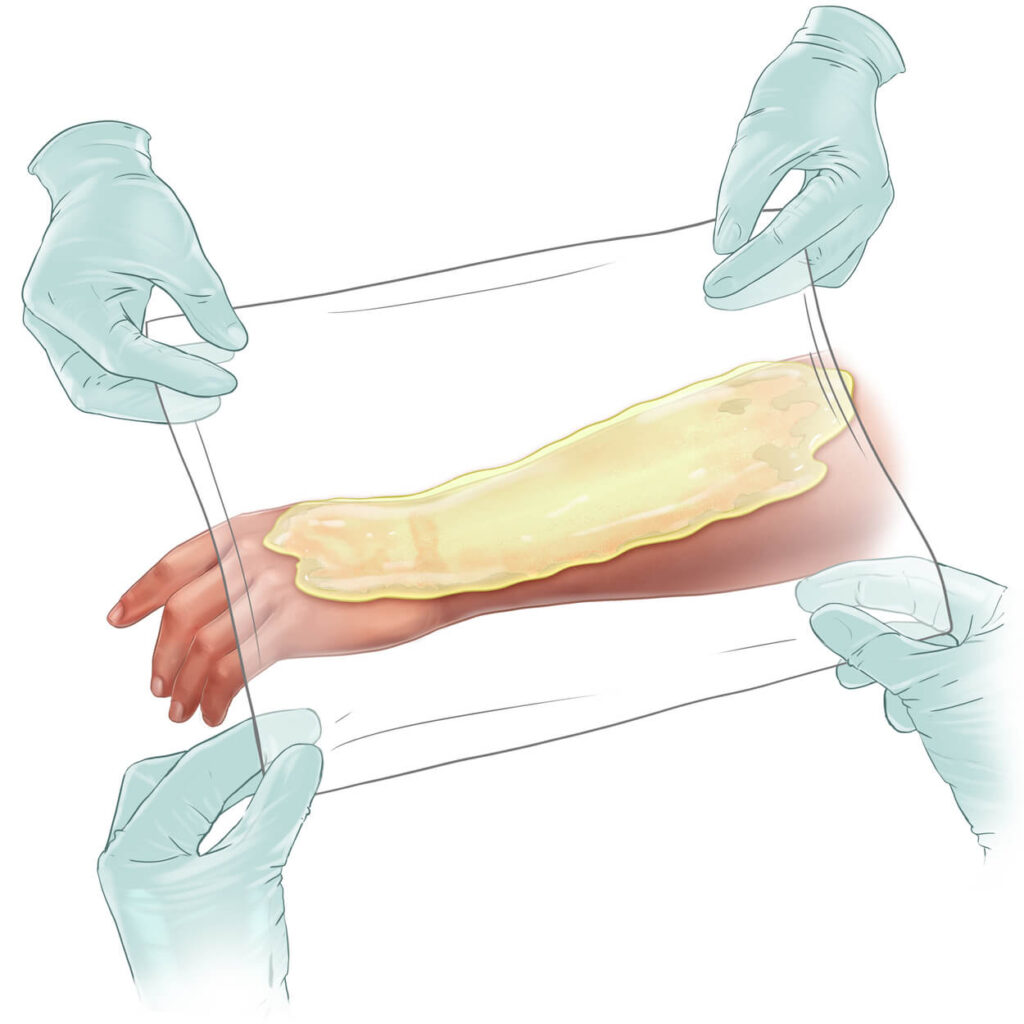
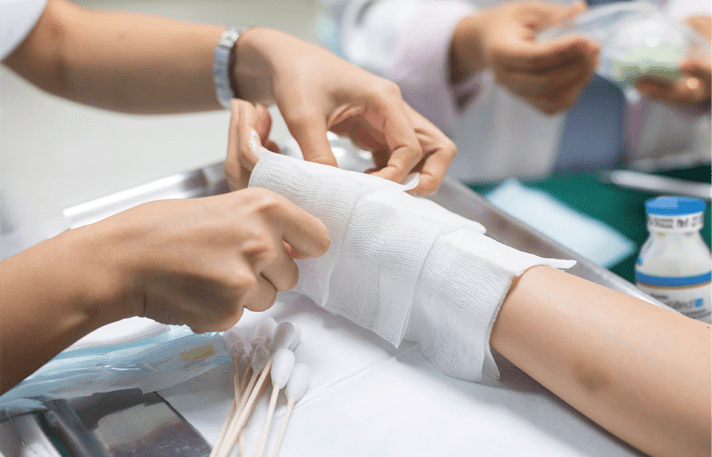
NexoBrid® is a topically administered, biological orphan drug for the enzymatic removal of eschar in patients with deep partial- and full-thickness thermal burns. It selectively removes non-viable tissue while preserving viable tissue and is approved for use in adults and pediatric patients in over 40 countries, including the United States, European Union, and Japan.
NexoBrid development has been supported with federal funds from the Biomedical Advanced Research and Development Authority (BARDA), under contract HHSO100201500035C.
NexoBrid development has been supported in whole or in part with federal funds from the U.S. Department of Health and Human Services; Administration for Strategic Preparedness and Response; Biomedical Advanced Research and Development Authority (BARDA)